Despite the frequency of these neonatal RBC transfusions, there is no universally accepted haemoglobin transfusion policy.
Anaemia of prematurity (AOP)
AOP is a multi-factorial condition defined by early (after birth) and significant anaemia that is associated with phlebotomy blood losses, lower erythropoietin (EPO) production and a limited bone marrow response.[2]
Diagnosis of AOP relies upon a combination of parameters such as non-specific clinical symptoms of anaemia as well as haemoglobin and haematocrit levels.[3, 4] However, the exact threshold for haemoglobin or haematocrit levels where inadequate tissue oxygenation (critical anaemia hypoxaemia) definitively occurs in either term or preterm infants is not determined. Overall, this makes the timing of transfusion a neonate extremely challenging.
Systematic reviews comparing liberal versus restrictive RBC transfusion triggers in neonates
Review 1. Whyte & Kirpalani 2011
- Included studies: Comparison of low (restrictive) versus high (liberal) haemoglobin levels, four randomised controlled trials, included VLBW infants only
- Primary review outcomes: mortality and/or morbidity (ROP grade 3 or greater, severe adverse findings on cranial ultrasound, chronic lung disease, adverse neurodevelopmental outcome)
- Results: No statistically significant difference in combined outcomes of death or serious morbidity at first hospital discharge (typical risk ratio 1.19; 95% CI 0.95-1.19), one trial only reported composite of adverse neurodevelopmental outcomes or death at 18-21 months corrected (no difference between groups)
- Conclusions: Use of restrictive compared to liberal transfusion thresholds in VLBW infants reduced transfusion exposure but was not associated with any difference is significant morbidity and/or mortality
- Comments: given the uncertainties of these conclusions, it would be prudent to avoid haemoglobin levels below the lower limits tested here
Review 2. Bassler et al 2008
- Included studies: Benefit to risk ratio of lower versus higher RBC transfusion thresholds, seven randomised controlled studies, included preterm infants ≤ 32 weeks gestation only
- Primary review outcomes: apnoea outcomes, ventilation and pulmonary status, ROP, growth, neurologic outcomes, mortality and/or survival with CLD, severe ROP or brain injury, length of stay, blood culture positive sepsis, bowel perforation, NEC, PDA treatment
- Results: Adverse outcomes were only described for individual studies and no attempt was made to combine results
- Conclusions: Clinical and methodological heterogeneity between studies prevented the authors from pooling any results beyond describing outcomes from individual studies
- Comments: Thorough descriptive summary of the individual study findings only
Review 3. Ibrahim et al 2013
- Included studies: Comparison of low (restrictive) versus high (liberal) haemoglobin levels, three randomised controlled studies, included VLBW infants only
- Primary review outcomes: brain injury on cranial ultrasound, ROP, CLD, NEC or death
- Results: No statistically significant differences found between groups for certain morbidities or mortality
- Conclusions: Restrictive transfusion thresholds may be used without an associated increased morbidity and/or mortality
- Comments: Findings consistent with earlier publications
Review 4. Venkatesh et al 2012
- Included studies: Twenty-seven randomised controlled trial included and grouped into:
- Trials comparing RBC transfusion and no transfusion/placebo (n=3); different thresholds for transfusion (n=6); differing doses or administration schedule (n=4) or different types/products of RBCs (n=14). Included neonates (preterm/term) <28 days of age at enrolment.
- Primary review outcomes: mortality, neurodevelopmental outcome and chronic lung disease
- Results: Trials comparing different transfusion thresholds: no significant differences in mortality (RR 1.22, 95% CI 0.84-1.75) or CLD were found
- Conclusions: many trials failed to report on outcomes that would considered of primary importance to clinicians with consistent reporting of adverse events is required
There is insufficient evidence to either support or disprove a restrictive or liberal RBC transfusion strategy in preterm or term neonates.
However, having a local blood transfusion policy and auditing practice has been shown to reduce the number of neonatal RBC transfusions without any associated increase in adverse outcomes.[15]
When to transfuse?
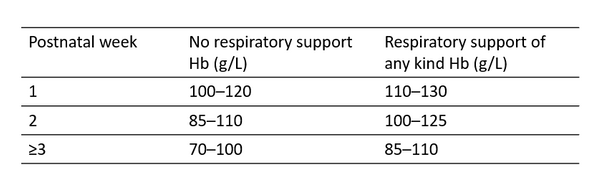
Whilst there is no evidence determining when to transfuse preterm and term infants, some degree of guidance is required to assist healthcare professionals. Below are suggested pragmatic transfusion thresholds for preterm and term neonates (Table).
Haemoglobin thresholds for transfusion in preterm and term neonates
References
- Temporal changes in blood product usage in preterm neonates born at less than 30 weeks' gestation in Canada A.K. Keir, J. Yang, A. Harrison, E. Pelausa, P.S. Shah. Transfusion 2015; 55:1340-1346.
- Anaemia of prematurity: pathophysiology and treatment, R.G. Strauss. Blood reviews 2010; 24:221-225.
- British Commitee for Standards in Haematology Transfusion Task Force: Writing. Transfusion guidelines for neonates and older children, B.E. Gibson, A. Todd, I. Roberts, D. Pamphilon, C. Rodeck, P. Bolton-Maggs, G. Burbin, J. Duguid, F. Boulton, H. Cohen, N. Smith, D.B. McClelland, M. Rowley, G. Turner, g. Br J Haematol 2004; 124:433-453.
- Practice guidelines for blood transfusion. American Red Cross; 2007. Miller Y, Bachowski G, Benjamin R. American Red Cross
- Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants R. Whyte, H. KirpalaniCochrane Database Syst Rev 2011:CD000512.
- Restrictive versus liberal red blood cell transfusion strategies for preterm infants: a systematic review of randomized controlled trials D.W. Bassler, Marcus; Bialkowski, Anja; Poets, Christian FCurrent Pediatric Reviews 2008; 4:143-150.
- Restrictive versus liberal red blood cell transfusion thresholds in very low birth weight infants: a systematic review and meta-analysis M. Ibrahim, S.K. Ho, C.L. Yeo.J Paediatr Child Health 2014; 50:122-130.
- The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trialsV. Venkatesh, R. Khan, A. Curley, S. Hopewell, C. Doree, S. StanworthBr J Haematol 2012; 158:370-385.
- Transfusion strategies for patients in pediatric intensive care units J. Lacroix, P.C. Hebert, J.S. Hutchison, H.A. Hume, M. Tucci, T. Ducruet, F. Gauvin, J.P. Collet, B.J. Toledano, P. Robillard, A. Joffe, D. Biarent, K. Meert, M.J. Peters, T. Investigators, G. Canadian Critical Care Trials, I. Pediatric Acute LungN Engl J Med 2007; 356:1609-1619.
- Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial D.A.Fergusson, P.Hebert, D.L.Hogan, L.LeBel, N.Rouvinez-Bouali, J.A.Smyth, K.Sankaran, A.Tinmouth, M.A.Blajchman, L.Kovacs, C.Lachance, S.Lee, C.R.Walker, B.Hutton, R.Ducharme, K.Balchin, T.Ramsay, J.C.Ford, A.Kakadekar, K.Ramesh, S.ShapiroJama 2012; 308:1443-1451.
- Furosemide for packed red cell transfusion in preterm infants: a randomized controlled trial V.K. Balegar, M. KluckowJ Pediatr 2011; 159:913-918 e911.
- Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants A. Ohlsson, S.M. AherCochrane Database Syst Rev 2014; 4:CD004863.
Relevant guidelines
- Guidelines on transfusion for foetuses, neonates and older children British Committee for Standards in Haematology 2016
- Red blood cell transfusion in newborn infants Canadian Paediatric Society Guidelines 2014
- Module 6 Neonatal and Paediatrics National Blood Authority (Australia) 2016
The author














