The Blood services wider contribution to public health? session included the following presentations:
1. Mike Busch: mportant role for biorepositories in global surveillance and epidemiological studies
2. Khoa Manh Dinh: The effect of COVID-19 interventions on virus nasal carriage among Danish blood donors
3. Bertram Kjerulff: Influence of sex, age, BMI, and smoking on 47 circulating inflammatory and vascular stress biomarkers in 9,876 healthy individuals – Results from the Danish Blood Donor Study
4. Mars Stone: Development of a Nationwide Repeat Blood Donor Cohort to Monitor SARS-CoV-2 Serosurveillance and Population Immunity
5. Mart Janssen: Generating synthetic blood transfusion data for haemoglobin deferral prediction
MODERATORS: Antoine Lewin, Ole Birger Pedersen
After the presentation, there was a questions and answers session, which is also included in the recording.
Abstract
Development of a nationwide repeat blood donor cohort to monitor SARS-CoV-2 serosurveillance and population immunity
M Stone1,2, E Grebe1, P Saa3, R Fink4, I Manrique4, C Di Germanio1, M Bravo5, V Green6, E Yu1, R Bruhn1,2, B Spencer3, E Notari3, M Lanteri6, S Stramer3, B Custer1,2, J Opsomer4, M Coughlin7, M Briggs-Hagen8, J Jones7, M Busch1,2
1Vitalant Research Institute, 2Laboratory Medicine, University of California, San Francisco, San Francisco, 3Scientific Affairs, American Red Cross, 4Westat, Rockville, 5Vitalant, Scottsdale, 6Creative Testing Solutions, Tempe, 7COVID-19 Response Team, Centers for Disease Control and Prevention, Atlanta, United States, 8COVID-19 Response Team, Centers for Disease Control and Prevention, Atlanta, United Arab Emirates
Background: Estimating population-level SARS-CoV-2 incidence, including vaccine breakthrough infections (VBTI) and reinfections (RI), is critical for public health surveillance. The prevalence of the population with a history of previous infection and the incidence of reinfections have both increased. Repeat, cross-sectional seroprevalence studies cannot estimate the incidence of reinfections.
Aims: We describe the objective and development of the first, nationwide repeat blood donor cohort (RDC); the RDC enables analyses of evolving SARS-CoV-2 infections and immunity in the U.S. population.
Methods: In this prospective cohort study, Vitalant and American Red Cross repeat blood donors were selected for inclusion in the RDC based on anti-spike (S) and anti-nucleocapsid (NC) serological testing results and reported COVID-19 vaccination history. During June 2020-July 2021, all blood donations were tested for SARS-CoV-2 antibodies and reactive samples frozen and archived. RDC donors were categorized according to SARS-CoV-2 infection and COVID-19 vaccination status at Q2 2021 and each quarter in 2022. Subsequent to July 1, 2022, serum/plasma samples were prospectively captured, frozen and stored from routine donations by RDC donors, and single samples were randomly selected each quarter and assayed for antibodies (Ab) to SARS-CoV-2 S (Ortho VITROS Anti-SARS-CoV-2 IgG Quantitative test) and NC (Ortho VITROS Anti-SARS-CoV-2 Total N Antibody test). Donors provided self-reported detailed vaccination history, infection history and clinical outcomes in response to quarterly online surveys. Participants were categorized into four groups, based on infection and vaccination history; previous infection was defined as any positive anti-NC test and vaccination status was reported on routine donation history questionnaires.
Results: The RDC, comprised of 142,612 repeat donors, was created to enable the following objectives and analyses: (1) establish methods to identify and discriminate primary infections, VBTI, and RI using longitudinal antibody results; (2) estimate quarterly (Q) seroprevalence and incidence from Q2 2021 to Q4 2022, weighted to better represent the US population; (3) characterise antibody kinetics following SARS-CoV-2 infection and vaccination; (4) determine the effectiveness of vaccinations for protection from infection and symptomatic disease; and (5) evaluate quantitative binding antibodies as a correlate of protection against infection and severe disease. The table shows initial results of population-weighted seroprevalence by history of infection and vaccination status from Q2 2021 to Q2 2022. Among all donors, the prevalence of those with serological evidence of previous infection increased from 20.3% to 58.4%, 2/3 of whom had also been vaccinated (hybrid immunity).
PA24-L04 – Table 1
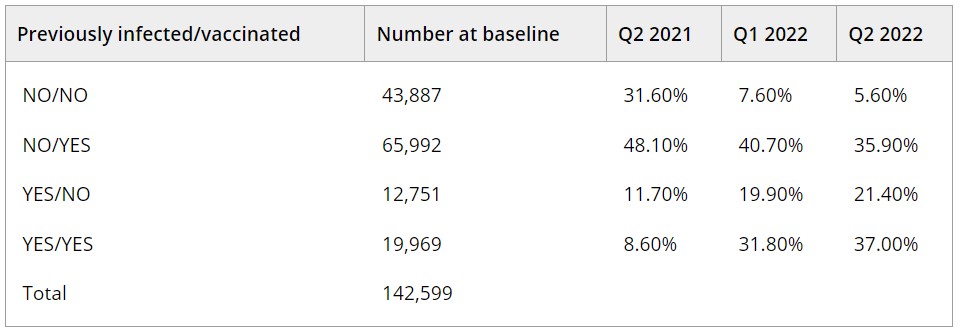
Table. Estimates of U.S. prevalence of previous SARS-CoV-2 infection and COVID-19 vaccination over time during Q2 2021–Q2 2022.
Q1: quarter 1 (Jan–Mar), Q2: quarter 2 (Apr–Jun)
Summary/Conclusions: This nationwide RDC is uniquely able to report U.S. estimates of evolving hybrid immunity which will include RI and VBTI rates. These data will be important to understanding the continued epidemic spread and evolving immunity to SARS-CoV-2 in the United States.


