The Epidemiology goes viral session included the following presentations:
1. Laura Tonnetti: The Impact of Babesia Testing on Transfusion-transmitted Babesiosis
2. Wen-Jie Liu: Ten-year experience of mini-pool nucleic acid testing in blood donors in Taiwan
3. Brian Custer: Prevalence, Incidence, and Residual Risk of HIV, HBV, and HCV in the US Blood Supply 2015 – 2021, on Behalf of the US TTIMS Program
4. Eduard Grebe: HIV incidence in US first-time blood donors during 12-month and 3-month MSM deferral policy periods on behalf of the US TTIMS Program
5. Daniel Candotti: Ten years insight into HBV molecular epidemiology in infected blood donors in Dalian, Northeast China
MODERATORS: Dudi Levi, Dorte Holm
After the presentation, there was a questions and answers session, which is also included in the recording.
Abstract
HIV incidence in US first-time blood donors during 12-month and 3-month MSM deferral policy periods on behalf of the US TTIMS program
E Grebe1, C Di Germanio1, M Stone1, E Notari2, R Bruhn1, M Lanteri3, D Kessler4, R Reik5, S Stramer2, M Busch1, B Hailu6, S Anderson7, B Whitaker7, B Custer1
1Vitalant Research Institute, San Francisco, CA, 2American Red Cross, Gaithersburg, MD, 3Creative Testing Solutions, Tempe, AZ, 4New York Blood Center, New York, NY, 5OneBlood, St Petersburg, FL, 6National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, 7Food and Drug Administration, Silver Spring, MD, United States
Background: Following FDA guidance, in 2016 US blood collection organizations implemented a 12-month time-limited deferral of male donors who reported sex with another man (MSM12m), and in 2020, further reduced to 3 months since last MSM sex (MSM3m). Here, we evaluate the incidence of HIV in first-time donors (FTD) using a recent infection testing algorithm (RITA) applied to HIV-infected donors at four large US blood collectors using data on 8.9 million donations.
Aims: The aims are to assess changes in HIV incidence in US FTD following eligibility policy changes and to inform assessment of HIV transfusion-transmission risk in the US blood supply.
Methods: We estimated HIV incidence and corresponding rate differences in FTD during the MSM12m and MSM3m periods, using cross-sectional incidence estimation techniques, based on application of a RITA to HIV-positive donations, as follows. NAT-yield donations (seronegative) were considered recent; Ab+/NAT- were considered long-term and concordant Ab+/NAT+ were classified as recent or long-term using quantitative Limiting Antigen (LAg) Avidity enzyme immunoassay (EIA) and viral load tests. HIV prevalence, a key input to the incidence calculation, was estimated through imputation to account for any missing confirmatory serology and/or NAT results. Prevalence and incidence confidence intervals were derived from parametric bootstrapping. Using a previously described Bayesian method, we estimated a context-specific mean duration of recent infection for the RITA using repeat donor data (243 days) and obtained a subtype B-specific false-recent rate estimate from external data. Given a shift in donor demographics associated with the COVID-19 pandemic, we assessed the impact of this shift on HIV incidence through a counterfactual age, sex and race/ethnicity standardised MSM3m estimate; that is, we weighted donors according to the number of donors in each age/sex/race-ethnicity stratum who donated during the MSM12m period. We used Poisson regression to assess demographic correlates of incident infection.
Results: FTD HIV prevalence during the MSM12m period was 8.59 cases/105 (95% CI: 7.79, 9.43) and during the MSM3m period was 9.13 cases/105 (8.01, 10.28). FTD HIV incidence during the two periods was 2.74 cases/105 person-years [PY] (2.05, 3.56) and 1.85 cases/105 PY (0.96, 2.85), respectively. The incidence point estimate declined substantially, but the difference was not statistically significant. The demographically standardised analysis did not demonstrate a similar decline (see Table). Multivariable regression indicated that male sex, younger age, Black or African American race, Hispanic ethnicity and residing in the Southern US were significantly associated with FTD incident infection.
PA18-L04 – Table 1
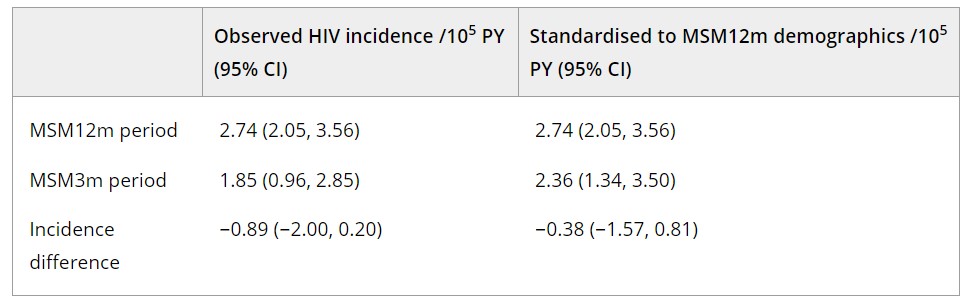
Summary/Conclusions: The decline in FTD HIV incidence from the MSM12m to MSM3m periods did not reach statistical significance (95% CI upper bound, increase of 0.2/105PY). The standardised analysis provides evidence that the decline may be partially explained by demographic shifts in the donor population. Similar demographic factors were associated with incident infection as in previous analyses. These results provide no indication of increased risk in FTD and are reassuring in that reduced deferral periods have not led to increased risk.






