The Crossmatching 2.0 session included the following presentations:
1. Christine Lomas Francis: CD47 Biotherapy: A new incompatibility threat
2. Tae-Shin Kim: The effects of IMC-002, a novel anti-CD47 monoclonal antibody, on pretransfusion compatibility testing
3. Wenhui Li: CD71+ RBCs Mediate Increased Erythrophagocytosis and Reduced Monocytes in Human Suspension Assay
4. Ling Wei: Evaluating the clinical significance of alloantibodies against GP.Mur using flow cytometry phagocytosis assay
5. Anne Cornelissen: Detection of erythrocyte alloantibodies in patient sera using a novel flow cytometry approach
MODERATORS: Sofia Lejon Crottet, Roland Fiskesund
After the presentation, there was a questions and answers session, which is also included in the recording.
Abstract
The effects of IMC-002, a novel anti-CD47 monoclonal antibody, on pretransfusion compatibility testing
T Kim1, Y Chung2, D Ko3, J Park1, H Kim1
1Department of Laboratory Medicine, Seoul National University Hospital, 2Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, 3Department of Laboratory Medicine, Asan Medical Center, Seoul, Republic of Korea
Background: Some therapeutic monoclonal antibodies currently used in clinical practice or trials target proteins expressed not only in cancer cells but also in normal cells, such as red blood cells (RBCs), raising concerns about their impact on pre-transfusion compatibility testing. CD47 is a glycosylated transmembrane protein with ubiquitous expression, including on RBCs. As tumour cells have been shown to highly express CD47 to evade phagocytosis by macrophages, many anti-CD47 monoclonal antibodies are being developed to treat cancers and hematologic malignancies. However, the evaluation of anti-CD47 monoclonal antibodies previously used in clinical trials showed variable interferences in pre-transfusion compatibility testing.
Aims: This study aims to investigate the effect of IMC-002, a novel anti-CD47 monoclonal antibody in clinical trials, on pre-transfusion compatibility testing.
Methods: Group O/RhD positive, AB/RhD positive, and AB/RhD negative EDTA whole blood samples were incubated with IMC-002 at final concentrations of 1000 and 2000 μg/mL at 37°C for 30 min. ABO/RhD typing was performed using the tube method, column agglutination technique (CAT) (ABO/D+ Reverse Grouping ID-Card, Bio-Rad), and an automated immunohematology analyser (Qwalys 3, Diagast). A1, H and extended blood group antigen testing were performed by the tube method. Direct antiglobulin testing (DAT) was performed using CAT (LISS/Coombs ID-Card, Bio-Rad). To simulate the plasma of patients receiving IMC-002, the drug was spiked into group AB/RhD positive plasma with no unexpected antibodies at concentrations of 0.1, 1, 10, 100, 500, 1000, and 2000 μg/mL. Antibody screening tests were performed using the tube method and CAT (LISS/Coombs ID-Card, Bio-Rad; DG Gel Card, Grifols) with R1R1, R2R2 and rr RBCs.
Results: Qwalys 3 showed false-positive results for ABO forward typing and RhD typing. Although manual tube and CAT methods for ABO forward typing and RhD typing using washed RBCs showed no interference, false-positive results were identified in ABO reverse typing. Saline replacement resolved the interference in ABO reverse typing for the tube method. DAT and A1, H, and extended blood group antigen testing using washed RBCs showed no interference. Panreactive interference was observed in antibody screening in both manual tube and CAT methods at plasma IMC-002 concentrations of ≥10 and ≥1 μg/mL, respectively (Table 1).
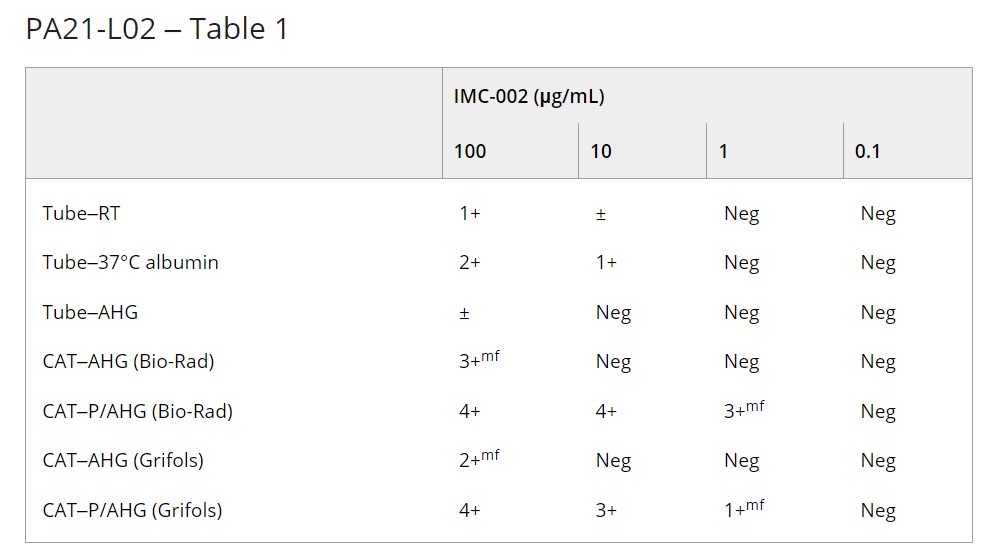
PA21-L02 – Table 1
Summary/Conclusions: Although IMC-002 showed interference in pre-transfusion testing the characteristics were distinct compared to other anti-CD47 monoclonal antibody drugs. The interference in ABO/RhD typing and antigen testing could be mitigated using washed RBCs and saline replacement. Panreactive results were observed in antibody screening at RT, 37°C and AHG phases with double populations in gel cards. However, the interference was not seen at relatively low drug concentrations.






















