Audits are an important component of a PBM strategy. In general, an audit involves checking a process, structure of even an outcome itself to ensure that it conforms to an expectation of performance. The audit will reveal whether the process is doing what is desired of it, and in an efficient manner. Audits of transfusion practice can occur both in the blood bank itself and on the hospital wards and operation rooms; audits of the latter are generally performed to ensure that clinicians are following the institutional directives or guidelines for transfusion practice. Simply by starting to look at a process, potential areas for improvement can be found. This text describes important audit topics both inside and outside the transfusion service, and includes some examples that can be adapted for use at your institution.
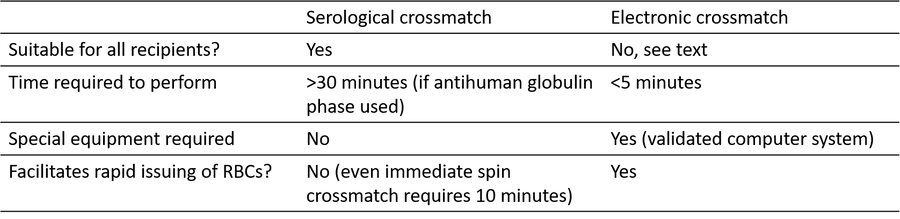
Table 1: Comparison of serological and electronic crossmatch technique
References
- Measuring trade-offs that matter: assessing the impact of a new electronic cross-match policy on the turnaround time and the cross-match workload efficiency. Lin DM et al.Transfusion 2014; 54 (12): 3075–3079.
- Effectiveness of multiple initiatives to reduce blood component wastage. Collins RA et al.Am J Clin Path 2015;143: 329-35.
- Evaluation of real-time clinical decision support systems for platelet and cryoprecipitate orders. Collins RA et al.Am J Clin Path 2014;141: 78-84.
- Effectiveness of a real-time clinical decision support system for computerized physician order entry of plasma orders. Yazer MH et al.Transfusion 2013; 53 (12): 3120–3127.
- Trends in RBC Ordering and Use After Implementing Adaptive Alerts in the Electronic Computerized Physician Order Entry System. McWilliams B et al.Am J Clin Path 2014;141: 534-41.
- Blood utilization after primary total joint arthroplasty in a large hospital network. Chen AF et al.HSS J: musculoskel J Hosp Spec Surg 2013;9: 123-8.
- Clinical validation of risk stratification criteria for peripartum hemorrhage. Dilla AJ et al.Obst Gyn 2013;122: 120-6.
- Red Blood Cell Salvage During Obstetric Hemorrhage. Milne ME et al.Obst Gyn 2015; 125 (4): 919-923.
Appendix
The obstetric service suddenly began ordering significantly more crossmatches than they had previously been ordering, which was noted at the author’s institution.
This increased crossmatch workload placed a burden on the transfusion service’s staff and increased the hospital’s blood charges. The transfusion service, through the hospital’s transfusion committee, discovered that a new guideline that dictated when antepartum patients should have a type and screen performed and when RBCs should be crossmatched had been implemented by the nursing staff in the labour and delivery ward. The transfusion service then conducted an audit to determine if these newly implemented guidelines were actually accurate in predicting the transfusion needs of these patients, and it turned out that many of the patients who had some form of pre-transfusion testing ordered because of the implementation of these guidelines did not require a transfusion at all! Only 2% of the women on which the guidelines predicted a moderate risk of experiencing a postpartum hemorrhage actually required at least 1 RBC, meaning that over 2400 type and screens were unnecessarily performed over a 1 year period amongst the women in this risk category [7]. Amongst the women in the high risk category during the same time period, where the guidelines suggested that not only should a type and screen be ordered but also some RBCs should be crossmatched, 7.3% of the women required 1 RBC unit leading to the transfusion service unnecessarily having performed a significant amount of pre-transfusion testing on nearly 400 additional women [7]. This audit was essential in developing new antenatal pre-transfusion testing guidelines that better reflected the local practice. This effort resulted in the changing of the pre-transfusion testing ordering habits of the obstetricians thereby relieving the workload burden on the transfusion service, reducing unnecessary testing for the patients, and lowering healthcare costs for the hospital system.
A 2nd 8.5 year long year long audit of the obstetrical service focused on intraoperative cell salvage
(ICS) in obstetrical cases [8]. This audit discovered that the vast majority of patients on which ICS was employed, 79%, did not actually receive a reinfusion of the salvaged blood because these patients did not shed enough blood during their case for ICS processing and reinfusion to occur. Cases where ICS is set up but no blood is reinfused due to the low volume of shed blood are known as stand by cases, and there is a fixed cost to these cases in terms of the hardware and disposables that are needed to collect whatever blood is shed, as well as the costs of the technologists to operate the machine. This audit revealed that the most efficacious use of ICS was in patients who underwent a Cesarean hysterectomy or who had some form of postpartum hemorrhage [8]. The results of this audit have also helped to shape the practice of obstetrics at this hospital.
The author
















